Learn about Alto Neuroscience's recent upsized IPO pricing, their innovative approach to drug development, and their future plans for developing personalized treatments. Find out how their AI-derived brain biomarkers are revolutionizing psychiatric drug development.
Alto Neuroscience IPO Pricing
Alto Neuroscience, Inc. recently announced the pricing of its upsized initial public offering (IPO) of 8,040,000 shares of common stock at a public offering price of $16.00 per share.
The company initially suggested a price range of $14-$16, but ultimately settled on $16.00 per share.
The aggregate gross proceeds from the IPO are expected to be approximately $128.6 million before deducting underwriting discounts, commissions, and other offering expenses.
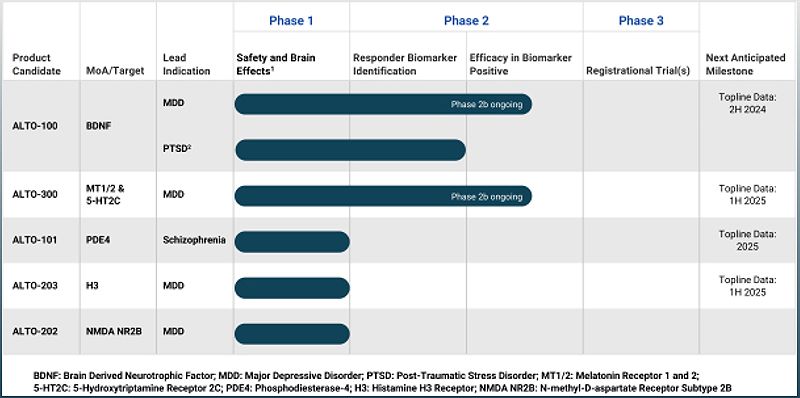
These funds will be used to further develop Alto Neuroscience's innovative drug candidates, Alto-100 and Alto-300.
Alto Neuroscience's Approach to Drug Development
Alto Neuroscience takes a unique approach to psychiatric drug development by leveraging neurobiology and AI-derived brain biomarkers.
By matching the right patient with the right Alto drug based on these biomarkers, the company aims to develop personalized and highly effective treatment options.
The company has successfully completed Phase 2a trials for its two most advanced product candidates, ALTO-100 and ALTO-300, in more than 200 patients each.
These trials identified patient populations more likely to respond based on objectively defined biomarker profiles.
Alto Neuroscience has also initiated a placebo-controlled, double-blind, randomized Phase 2b trial for each candidate in patients with Major Depressive Disorder (MDD) characterized by an objective biomarker.
Future Plans for Drug Development
In addition to their advanced programs, Alto Neuroscience has plans to initiate Phase 2 proof-of-concept trials for ALTO-101 and ALTO-203 in the first half of 2024.
ALTO-101 is being developed for patients with cognitive impairment associated with schizophrenia (CIAS), while ALTO-203 is being developed for patients with MDD and higher levels of anhedonia (lack of motivation or pleasure).
The company expects to report topline data from these trials in 2025 and the first half of 2025, respectively.
Alto Neuroscience also plans to develop ALTO-202, a novel oral N-methyl-D-aspartate (NMDA) receptor antagonist, for the treatment of patients with MDD.
Financial Overview and Challenges
Alto Neuroscience has primarily funded its operations through proceeds from the sales of convertible preferred stock and borrowings under its loan and security agreement.
As of September 30, 2023, the company had an accumulated deficit of $65.7 million and had not generated any revenue from product sales.
While the company raised additional net proceeds of approximately $44.4 million through the issuance and sale of its Series C convertible preferred stock in November 2023, it expects to continue generating operating losses and negative operating cash flows for the foreseeable future.
However, with its successful IPO and ongoing clinical trials, Alto Neuroscience is well-positioned to pursue its drug development goals.