In a groundbreaking study, researchers from the Universitat Autònoma de Barcelona, the Karolinska Institute, and BioArctic have revolutionized our understanding of Alzheimer's disease. By harnessing the power of STED microscopy, a cutting-edge technology that allows for super-resolution visualization, and a newly developed antibody, they have unlocked the secrets of amyloid aggregates, the hallmark of the disease. This breakthrough surpasses the limitations of conventional microscopy, enabling us to delve deeper into the structure and formation of these aggregates. Join us as we embark on a journey into the hidden world of Alzheimer's disease and explore the implications of this groundbreaking research.
Unveiling the Structure of Amyloid Aggregates
Explore the groundbreaking use of STED microscopy and a new antibody to visualize the structure and morphology of amyloid aggregates in Alzheimer's disease.
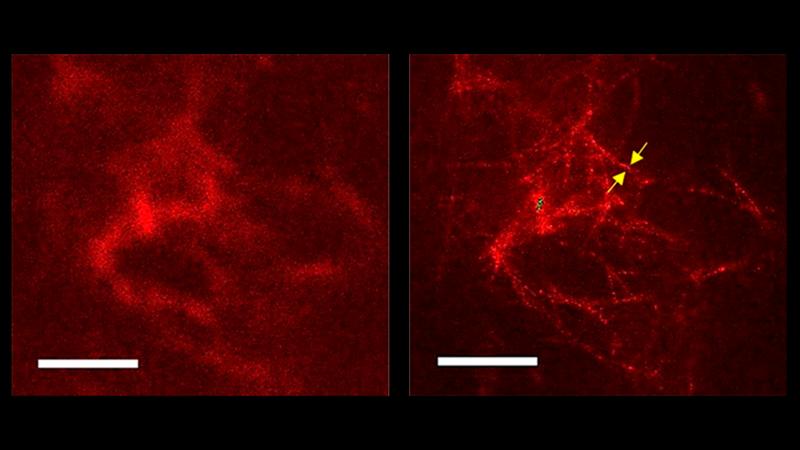
In the brains of Alzheimer's disease patients, the accumulation of plaques made up of beta-amyloid (Aβ) protein aggregates is a key pathological feature. However, conventional confocal light microscopy has only provided limited information about the structure of these aggregates. Enter STED microscopy, a cutting-edge technology that surpasses the capabilities of confocal microscopy and allows for super-resolution visualization.
By combining STED microscopy with a newly developed antibody that selectively reacts with Aβ aggregates, researchers have achieved a spatial resolution that exceeds that of conventional microscopy. This breakthrough has enabled them to discern individual fibers within the plaques, a milestone previously only possible with electron microscopy. The ability to visualize the interwoven fibril-like structure of amyloid aggregates opens up new avenues for studying the mechanisms involved in their formation and removal.
The Power of STED Microscopy
Discover the capabilities of STED microscopy in surpassing the limitations of conventional confocal microscopy for studying amyloid aggregates in Alzheimer's disease.
STED microscopy, developed by Nobel laureate SW Hell, utilizes a stimulated emission depletion technique to achieve super-resolution imaging. Unlike confocal microscopy, which relies on a pinhole to eliminate out-of-focus light, STED microscopy uses a donut-shaped depletion beam to suppress fluorescence from the outer regions of the excitation spot. This allows for sharper and more detailed imaging of structures, such as amyloid aggregates, that were previously beyond the reach of conventional microscopy.
With STED microscopy, researchers have been able to visualize the individual fibers within amyloid plaques, providing unprecedented insights into their structure and morphology. This technology has the potential to revolutionize Alzheimer's research by shedding light on the mechanisms underlying plaque formation and clearance.
A New Antibody for Selective Visualization
Learn about the development of a new recombinant human antibody that selectively reacts with amyloid aggregates, enhancing their visualization in Alzheimer's disease.
In addition to STED microscopy, the researchers utilized a newly developed recombinant human antibody labeled with fluorescence to selectively react with amyloid aggregates. This antibody specifically targets the Aβ protein, allowing for enhanced visualization of the aggregates in brain tissue sections.
The combination of STED microscopy and the new antibody has provided researchers with a powerful tool to study the structure and morphology of amyloid aggregates in Alzheimer's disease. By observing the interwoven fibril-like structure of the plaques, researchers can gain a deeper understanding of the mechanisms involved in their formation and potentially identify targets for therapeutic interventions.
Unraveling the Mysteries of Alzheimer's Disease
Delve into the implications of this groundbreaking research for unraveling the mysteries of Alzheimer's disease and advancing our understanding of its development and progression.
Alzheimer's disease is a complex neurodegenerative disorder characterized by the accumulation of amyloid plaques and neurofibrillary tangles in the brain. Despite decades of research, many questions remain unanswered about the underlying mechanisms and progression of the disease.
The breakthrough use of STED microscopy and the new antibody provides researchers with a powerful tool to investigate the structure and formation of amyloid aggregates, shedding light on their role in the pathogenesis of Alzheimer's disease. This knowledge could pave the way for the development of novel therapeutic strategies and interventions aimed at preventing or slowing down the progression of the disease.
Furthermore, the ability to visualize amyloid aggregates in animal models under behavioral observation opens up new possibilities for studying the relationship between cognitive and neuropsychiatric symptoms and the development of Alzheimer's disease. By bridging the gap between behavioral observations and neuropathological correlates, researchers can gain valuable insights into the disease's progression and potentially identify early biomarkers for diagnosis and intervention.
Conclusion
The combination of STED microscopy and a new antibody has revolutionized our understanding of Alzheimer's disease and the structure of amyloid aggregates. By surpassing the limitations of conventional confocal microscopy, researchers have been able to visualize the interwoven fibril-like structure of amyloid plaques at an unprecedented level of detail. This breakthrough opens up new avenues for studying the mechanisms involved in plaque formation and clearance, ultimately leading to potential therapeutic interventions.
Furthermore, the ability to observe amyloid aggregates in animal models under behavioral observation provides valuable insights into the development and progression of cognitive and neuropsychiatric symptoms in Alzheimer's disease. This research has the potential to uncover early biomarkers and improve diagnostic and intervention strategies.
FQA :
What is STED microscopy?
STED microscopy is a super-resolution imaging technique that utilizes a stimulated emission depletion technique to achieve sharper and more detailed imaging of structures, such as amyloid aggregates in Alzheimer's disease.
What is the significance of visualizing the structure of amyloid aggregates?
Visualizing the structure of amyloid aggregates provides crucial insights into their formation and potential targets for therapeutic interventions in Alzheimer's disease.
How does the new antibody enhance visualization of amyloid aggregates?
The new recombinant human antibody selectively reacts with amyloid aggregates, allowing for enhanced visualization of these structures in brain tissue sections.
What are the implications of this research for Alzheimer's disease?
This research has the potential to advance our understanding of Alzheimer's disease, unravel its mysteries, and pave the way for the development of novel therapeutic strategies.